In regulated life sciences environments, the management of controlled documents such as SOPs (Standard Operating Procedures), procedural instructions or work instructions is of great importance. Change management processes ensure that these documents are properly revised, approved, trained, distributed and, where necessary, suspended. In addition to well-known use cases within change management, there are special cases that are handled differently from company to company.
One of these applications is the case of the so called document release process.
Case Study
The work instruction for a new liquid chromatograph that allows substances to be physically separated undergoes an electronic approval process using an in-house document management system (DMS). Depending on the individual company guidelines, document coordinators, operations or quality managers sign the work instructions electronically. On October 1st, signing is successfully completed and the DMS ensures that the work instruction in the DMS becomes »Approved«. At the same time, the DMS changes the access rights to the document: write access is revoked and read access is granted exclusively to the document coordinators and quality representatives. – The document is now approved.
As in the case of many other critical types of documents, the personnel who will use the work instructions in the future work routine must now be trained accordingly: Not only the contents of the working instructions, but also the handling of the underlying liquid chromatograph must be extensively trained. Therefore, it is necessary to set up the device in a technical center after it has been purchased, for example, and to set up a test stand. The training has to be organized and the staff invited. All these preparations were completed on November 1st and a document coordinator changes the status of the work instruction in the DMS from »Approved« to »Released«. The DMS changes access rights again: In addition to document coordinators and quality representatives, the DMS now also grants read access to all training participants and later users. Every employee who goes through the training and will work with the work instructions in his or her day-to-day work can now view the document in the DMS or trigger a print process to familiarize himself or herself with the document. – The document is now released.
After several weeks the whole staff has passed through the training. The liquid chromatograph is installed in the respective laboratory and replaces, for example, an obsolete instrument. The laboratory manager, in cooperation with a quality manager, sets the deadline of 1st December: from this date on, the laboratory staff may only use the new device. At the same time, a document coordinator in the DMS defines December 1st as the effective date for the corresponding work instruction. On this key date, the DMS changes the document status from »Released« to »Effective«. The DMS also ensures through appropriate access rights that any previous versions of the work instructions are no longer visible to end users. Only the effective version of the document may be used from the key date on. Every printout that a user initiates from now on is automatically logged by the DMS (controlled printing). – The document is now effective.
The chronological sequence of the document release in this case example is therefore as follows:
October 1st: Document fully approved. All those responsible signed the document.
November 1st: Document released. The document can now be viewed by end users.
December 1st: Document effective. From now on the document must be used.
Solution from the user’s point of view (excerpt)
All signatories sign the document electronically within the DMS by means of an approval workflow. At the end of the approval process, a document coordinator determines the planned effective date. If this has not yet been determined at the time of approval, it can be determined at a later date (see below).
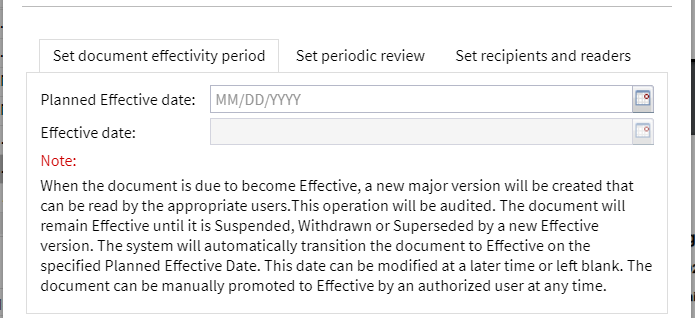
The document is now approved and presented accordingly.
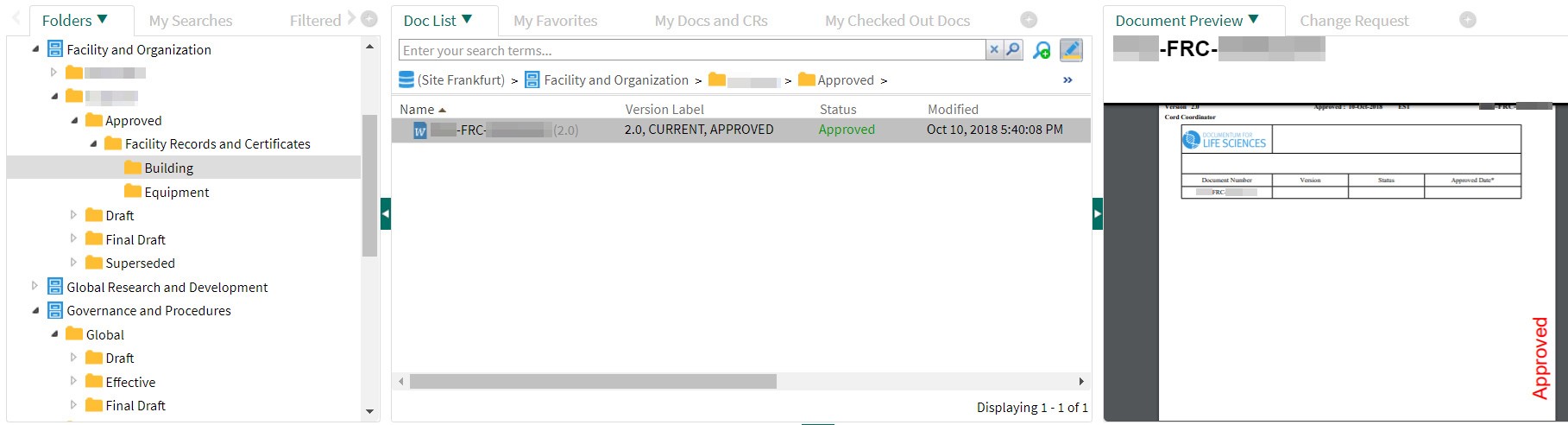
As soon as a document coordinator releases the document, the DMS changes the document status (»Released«) and adjusts the access rights accordingly:
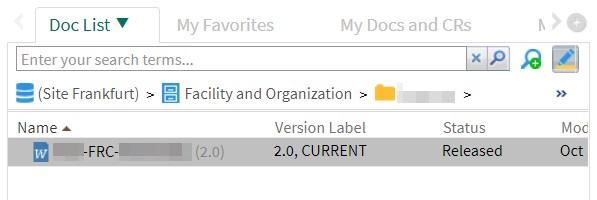
After successful training and completion of all preparations, the document coordinator determines the effective date. If the effective date has already been defined (see above), one can change it later. Alternatively, you can skip this step:
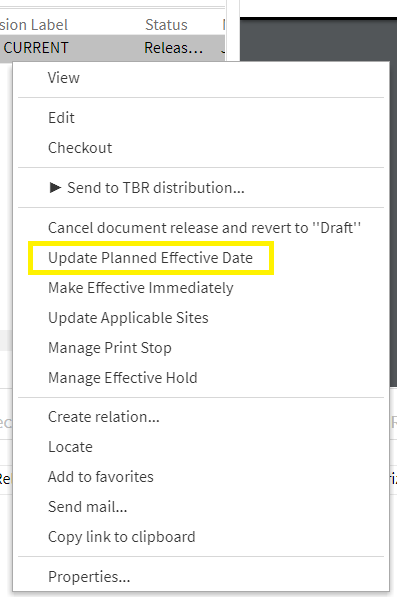
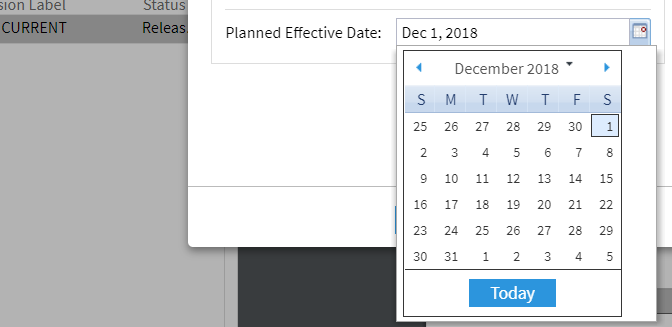
The DMS ensures that the document is transferred to the status »Effective« on December 1st, including changed access authorizations.
Technical implementation (excerpt)
The outlined business process can be implemented on the basis of OpenText Documentum for Life Sciences. The detailed customer requirements can be very different in this application.
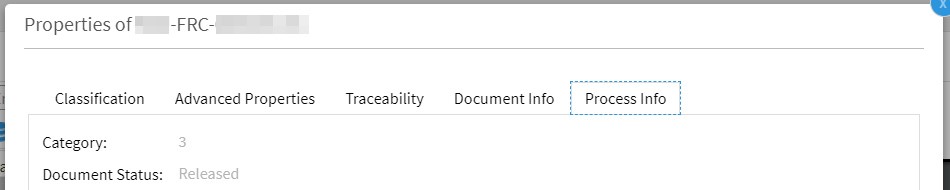
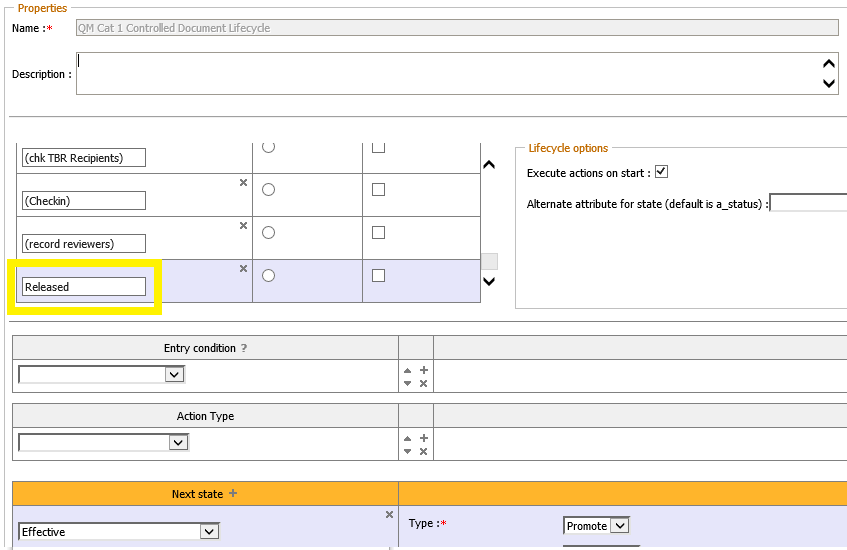
The OpenText Life Sciences solution provides a standard two-step approval process for Category 1 documents: A document is transferred to the effective status once it has been fully approved. A separate »Release« status between »Approved« and »Effective« does not exist. (Please note: Within the Life Sciences solution the »Approved« state is called »Release Pending«. The wording may be a little bit confusing, but »Release Pending« and »Released« must not be mixed up!)
Category 2- and Category 3 documents do not own a »effective« but only an »approved« status.
Depending on customer requirements, the »QM Cat 1 Controlled Document Lifecycle« must be adapted for technical implementation. An additional »Released« status can be added before the existing »Effective« status, but this measure requires very good knowledge of how the Life Sciences solution works.
Do you have any question or wish further information?
Simply fill out our contact form or visit our OpenText Documentum for Life Sciences Landingpage.
Here you can find all three parts of my blog post series »Special use cases based on OpenText Documentum for Life Sciences«:
The Extremely Efficient Effectivity Hold
The Mysterious Case of TDC
A Contribution to Controlled Printing





0 Comments